It sounds like something out of a sci-fi movie – and yet it’s a real scientific fact: water can boil and freeze at the exact same time. This rare condition isn’t a trick or paradox – and it’s known in physics as the triple point. It’s not something you’ll see in your kitchen, but it’s one of the most fascinating facts about water, and one that scientists rely on to define temperature itself.
Here’s how it works, where it happens, and why it matters.
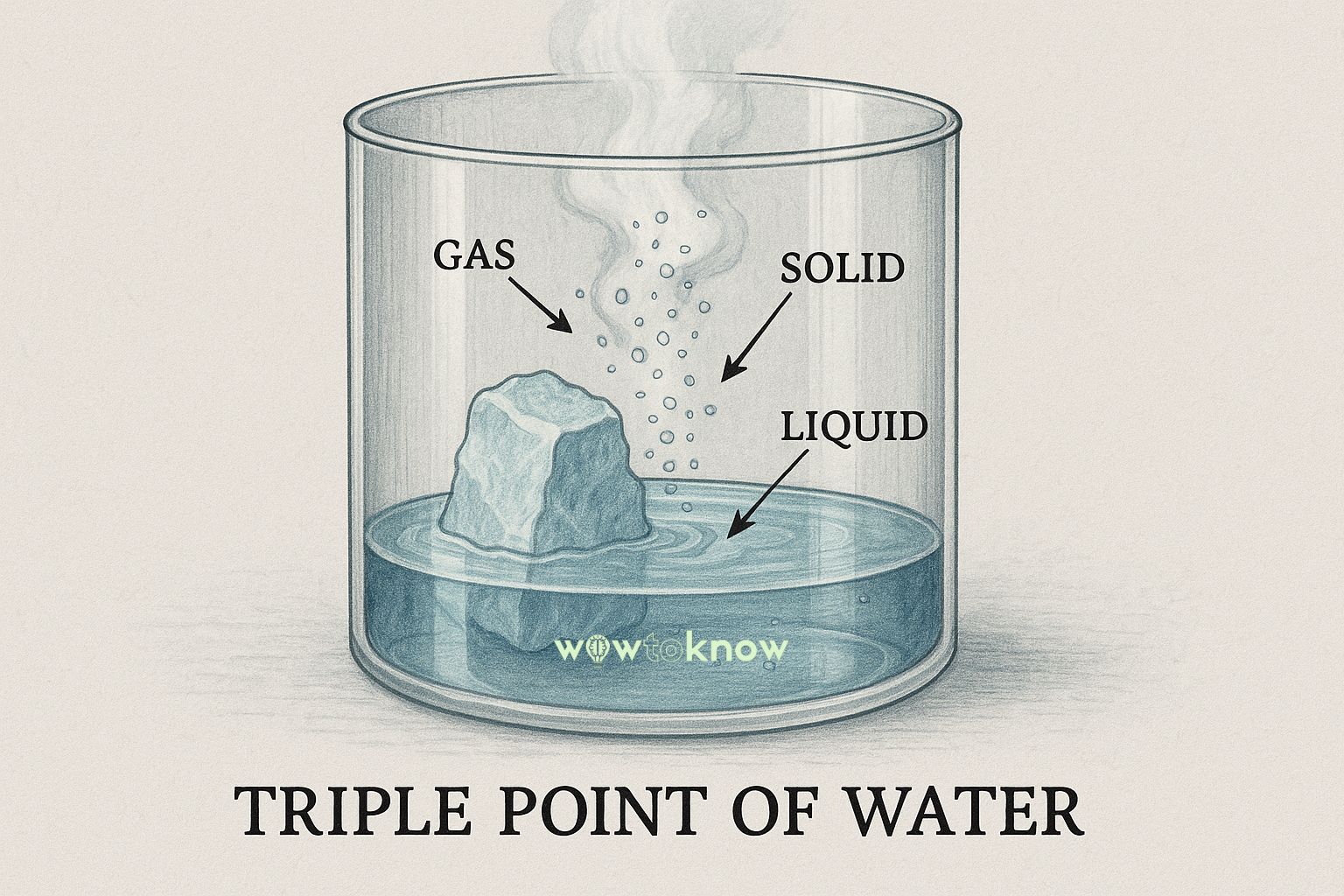
What Is the Triple Point of Water?
The triple point is a specific temperature and pressure at which a substance can exist simultaneously in three phases – solid, liquid, and gas.
For water, this point occurs at a temperature of 0.01°C (273.16 K) and a pressure of 611.657 pascals – that’s less than 1% of the atmospheric pressure at sea level. Under these precise conditions, ice, liquid water, and water vapor can coexist in equilibrium. Boiling and freezing are happening at the same time, and neither phase dominates.
The triple point is not unique to water – every pure substance has one. But water’s triple point is especially important. It’s used as a fixed point in thermodynamic temperature scales, and it’s even part of how the Kelvin scale is defined.
How Water Can Boil and Freeze at Once
Under normal conditions, boiling and freezing are opposite processes. You boil water by adding heat, and you freeze it by removing heat. But when pressure is reduced to the triple point, the phase boundaries blur. Water doesn’t need additional heat to boil, nor does it need extreme cold to freeze – it does both, because the energy in the system is evenly distributed across its three phases.
The result is a surreal visual: you can watch a droplet of water bubbling and forming ice crystals at the same time. It’s not a trick of the eye – it’s the laws of thermodynamics at work.
But this only happens in controlled laboratory environments. You can’t recreate it at home because you need specialized equipment like a vacuum chamber to drop the pressure low enough.
Real-World Demonstrations
One of the most popular visual demonstrations of water’s triple point comes from physics labs and educational science centers. Using a sealed vacuum chamber and a bit of distilled water, scientists reduce the pressure until the triple point is reached – and then watch as the water boils and freezes at the same time.
NASA has even documented the triple point in educational videos to show how water behaves in space environments. The triple point is a major consideration for scientists designing equipment for extraterrestrial missions, because planetary bodies like Mars have surface pressures that hover near water’s triple point. That means liquid water on Mars is inherently unstable – it either evaporates or freezes.
In precision labs, triple-point cells are used to calibrate thermometers to exact standards. These devices contain highly purified water at its triple point and are used around the world to maintain consistent temperature measurements.
You can watch a verified demonstration by physicists here:
Why This Matters in Science and Industry
The triple point of water isn’t just a cool experiment – and it’s a benchmark in thermodynamics. It plays a role in:
- Metrology – The science of measurement. The triple point of water is one of the fixed points used in defining the International Temperature Scale of 1990 (ITS-90).
- Cryogenics – Understanding phase changes helps scientists control the behavior of fluids at extremely low temperatures.
- Aerospace and planetary science – Water behaves very differently in space or on other planets. Knowing the triple point helps researchers predict how water-based systems will perform.
- Material science – Phase diagrams help chemists and engineers understand how substances behave under varying pressure and temperature.
In essence, the triple point gives scientists a tool for predicting, measuring, and engineering across fields.
Other Substances with Strange Triple Points
Water isn’t the only substance with a triple point, though it’s one of the most studied. Here are a few others with surprising behaviors:
- Carbon dioxide (CO₂) – At normal Earth pressures, CO₂ goes directly from solid to gas – a process known as sublimation. That’s why dry ice doesn’t melt into liquid under normal conditions.
- Helium – Its triple point is extremely low, close to absolute zero. Helium behaves unlike any other element and can even enter a superfluid state, flowing without friction.
- Sulfur – Has a triple point at high pressure and shows multiple solid phases, making its phase diagram unusually complex.
These substances help scientists explore everything from basic chemistry to exotic physics like quantum states.
FAQs
Can you see the triple point with your eyes?
Yes – and it’s been captured on camera many times. Under lab conditions, you can observe a small amount of water boiling and forming ice crystals at the same time. It’s a popular experiment in physics classes and science demos.
Can water do this naturally on Earth?
Not really. The pressure required for the triple point is extremely low – far below what’s naturally found on Earth’s surface. However, it’s possible that in high-altitude conditions or certain industrial vacuum environments, water might briefly approach triple point behavior.
Does this break the laws of physics?
Not at all. It proves them. The triple point is one of the best demonstrations of phase equilibrium and thermodynamics in action. It’s strange but completely consistent with established science.
Is this different from supercooled water?
Yes. Supercooled water is water that remains liquid below its normal freezing point. It happens because no crystal seed exists to start the freezing process. The triple point, by contrast, is a state where all three phases – solid, liquid, gas – are balanced and happening at once.
Can it happen in space or other planets?
Yes – and that’s part of why it’s important. On Mars, the atmospheric pressure is close to the triple point of water, which means liquid water can’t exist for long. It either freezes or boils away. That’s one reason scientists look for salty water (brine), which has a lower freezing point and might remain stable longer.
Final Thought: When Science Sounds Like Magic
Water boiling and freezing at the same time might sound like a trick – and but it’s one of the clearest examples of how strange and elegant physics can be. The triple point isn’t just a quirky fact – it’s a critical reference point used in labs, satellites, and scientific measurements around the world.
It reminds us that nature often has rules stranger than fiction – and those rules help us build everything from space probes to thermometers.
Fancy more wow-facts from the fascinating world of science? Did you know that one of the longest scientific experiments has been going on for nearly 100 years?To learn something new and amazing each day, follow WowToKnow on Facebook, X, Reddit, and Telegram.






minsk vacation packages Travelshop Booking delivered an exceptional travel day filled with beautiful sights and excellent service. Everything was arranged with great care, from the pick-up time to the final drop-off. The guide was knowledgeable and approachable, always ready to help and provide explanations. The pace of the tour felt perfect, allowing us to enjoy every moment without rushing. I genuinely admire the professionalism of this company and highly recommend their tours to others. https://americaneduforce.edu.vn/?p=2117
MG Bet, always a good time! Their site is easy to navigate and I’ve had some decent luck there. Check it out! mg bet.com
Logging into qq288login is quick and painless. No crazy hoops to jump through. I appreciate that they keep it simple and secure. Definitely makes getting in and playing a breeze. You can find it here: qq288login